Project P14 | Christian Ungermann
Plasticity and adaptation of the endosomal system
We focus on signaling endosome biogenesis in the endolysosomal system and clarify the role of the lipid kinase complex Fab1 and its regulation by the nutrient regulated TOR1 kinase complex.
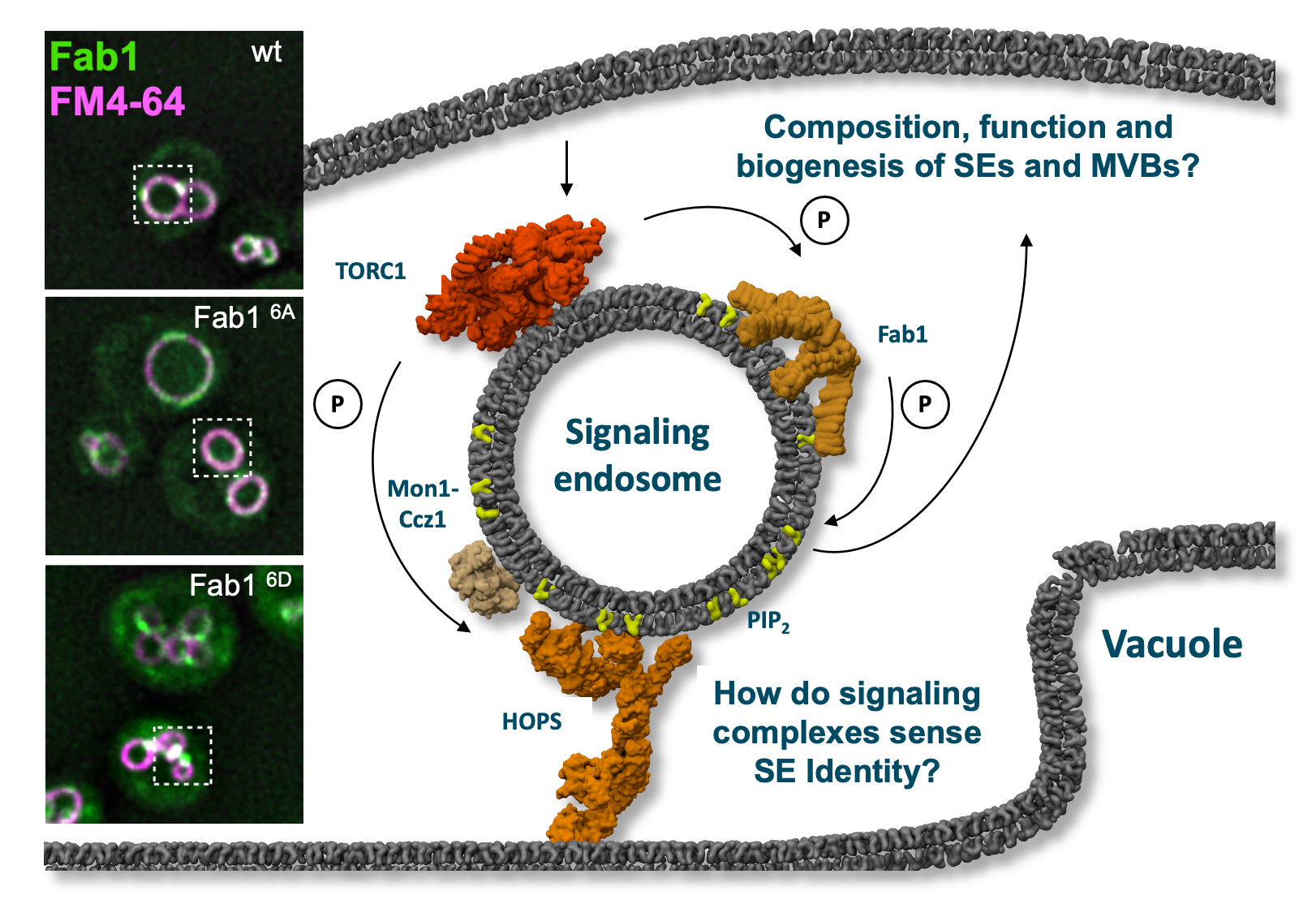
Signaling endodomes are endosomes proximal to the lysosome-like vacuole. They harbor, among others, the Fab1 lipid kinase and the TORC1 signaling complex. Fab1 can be phosphorylated by TORC1, which causes its relocalization to dots as shown in the bottom image on the left. A non-phospho mutant of Fab1 (6A) resides instead on vacuoles. Within project P14, the function of both kinases will be analyzed. © Christian Ungermann
Project Summary
Prof. Dr. Christian Ungermann
Osnabrück University
School of Biology/Chemistry
Research Group Biochemistry

The endolysosomal system plays a key role for membrane and membrane protein homeostasis and organelle quality control. During metabolic adaptations or stress, several plasma membrane proteins are transported to early endosomes (EE) and can then either be rerouted via recycling endosomes (REs) or get degraded in the lysosome. In the latter pathway, EE mature into late endosome, where proteins are sorted into intraluminal vesicles. These multivesicular bodies (MVBs) then fuse with lysosomes to deliver lipids and proteins for degradation into their building blocks for reuse.
Lysosomes, or their equivalent in yeast, the vacuole, carry the kinase Target Of Rapamycin Complex 1 (TORC1) on their surface. This metabolic master regulator responds to changes in nutrient levels, but also the membrane composition, and in turn controls synthesis, turnover and degradation of membrane components. TORC1 is also found on endosomes. These signaling endosomes (SEs) are distinct from MVBs.
We recently showed that the phosphatidylinositol-3-phosphate (PI3P) 5-kinase, termed Fab1 complex (FAB1C; in humans PIKfyve), which generated PI(3,5)P2 from PI3P, is a target of TORC1 on SEs. The change in the PIP composition results in altered membrane composition, shape and dynamics as multiple proteins, including TORC1, bind to these lipids.
Indeed, TORC1-mediated phosphorylation of Fab1 changes vacuole and endosome morphology, TORC1 signaling, and Fab1 localization, reflecting the functional plasticity of the endosomal membrane system in response to lipid and protein signals. As both TORC1 and FAB1C respond to multiple cellular stress signals, we postulate that their crosstalk regulates membrane homeostasis at SEs, MVBs and vacuoles, which will be explored in the project.
Project-related Publications
Gao, J., Nicastro, R., Péli-Gulli, M.-P., Grziwa, S., Chen, Z., Kurre, R., Piehler, J., De Virgilio, C., Fröhlich, F., Ungermann, C. (2022) The HOPS tethering complex is required to maintain signaling endosome identity and TORC1 activity. J Cell Biol 221, e2021090884.
Vargas Duarte, P., Hardenberg, R., Mari, M., Walter, S., Reggiori, F., Fröhlich, F., González Montoro, A., Ungermann, C. (2022) The yeast LYST homolog Bph1 is a Rab5 effector and prevents Atg8 lipidation at endosomes. J Cell Sci 135, jcs259421.
Chen, Z., Malia, P.C., Hatakeyama, R., Nicastro, R., Hu, Z., Péli-Gulli, M.-P., Gao, J., Nishimura, T., Eskes, E., Stefan, C.J., Winderickx, J., Dengjel, J., De Virgilio, C., Ungermann, C. (2021) TORC1 determines Fab1 signaling function at signaling endosomes and vacuoles. Current Biology, 31, 297-309.
Langemeyer, L., Borchers, A.-C., Hermann, E., Füllbrunn, N., Han, Y., Perz, A., Auffarth, K., Kümmel D., Ungermann, C. (2020) A conserved and regulated mechanism drives endosomal Rab transition. elife, 9, p191.
Schoppe, J., Mari, M., Yavavli, E., Auffarth, K., Cabrera, M., Walter, S., Fröhlich, F., Ungermann, C. (2020) AP-3 vesicle uncoating occurs after HOPS-dependent tethering. EMBO J, e105117.
Malia, P.C., Numrich, J., Nishimura, T., González Montoro, A., Stefan, C.J., Ungermann, C. (2018) Control of vacuole membrane homeostasis by a resident PI-3,5-kinase inhibitor. Proc Natl Acad Sci USA 115, 4684-4689.
Langemeyer, L., Perz, A., Kümmel, D., Ungermann, C. (2018) A guanine nucleotide exchange factor (GEF) limits Rab GTPase driven membrane fusion. J Biol Chem 293, 731–739.
D'Agostino, M., Risselada, H.J., Lürick, A., Ungermann, C., Mayer, A. (2017). A tethering complex drives the terminal stage of SNARE-dependent membrane fusion. Nature 551, 634–638.
Arlt, H., Auffarth, K., Kurre, R., Lisse, D., Piehler, J., Ungermann, C. (2015). Spatiotemporal dynamics of membrane remodeling and fusion proteins during endocytic transport. Mol Biol Cell 26, 1357–1370.
Numrich, J., Péli-Gulli, M.-P., Arlt, H., Sardu, A., Griffith, J., Levine, T., Engelbrecht-Vandré, S., Reggiori, F., De Virgilio, C., Ungermann, C. (2015) The I-BAR protein Ivy1 is an effector of the Rab7 GTPase Ypt7 involved in vacuole membrane homeostasis. J Cell Sci 128, 2278-92.